Back
Present
Facts for Kids
Osmosis is the spontaneous movement of water through a selectively-permeable membrane, balancing concentrations of solute on either side.

Explore the internet with AstroSafe
Search safely, manage screen time, and remove ads and inappropriate content with the AstroSafe Browser.
Download

Become a Creator with DIY.org
A safe online space featuring over 5,000 challenges to create, explore and learn in.
Learn more
Overview
Osmosis is a cool science process that happens all around us! 🌍
It’s how water moves through thin barriers, like cell membranes. Think of it like water taking a trip! When water comes from a place with lots of it (high water potential) to a place with not so much (low water potential) through a special kind of door (selectively-permeable membrane), it helps balance everything out. This balancing act is super important for living things, like plants and animals, to stay healthy and hydrated. So, the next time you sip water, remember, you’re enjoying a bit of osmosis magic! 💧✨
It’s how water moves through thin barriers, like cell membranes. Think of it like water taking a trip! When water comes from a place with lots of it (high water potential) to a place with not so much (low water potential) through a special kind of door (selectively-permeable membrane), it helps balance everything out. This balancing act is super important for living things, like plants and animals, to stay healthy and hydrated. So, the next time you sip water, remember, you’re enjoying a bit of osmosis magic! 💧✨
Read Less
Types of Osmosis
Osmosis can happen in different ways, but let’s focus on two main types! First, there’s hypertonic osmosis, where the outside has a lot of salt or sugar, so water leaves the cell. This can make the cell shrink! 😱
On the other side, hypotonic osmosis has less salt or sugar outside than inside the cell. So, water rushes in, making the cell swell, sometimes like a balloon! 🎈
Balancing these types is super important for cells. Knowing these helps scientists and gardeners understand how to take care of plants and animals! 🌻🐾
On the other side, hypotonic osmosis has less salt or sugar outside than inside the cell. So, water rushes in, making the cell swell, sometimes like a balloon! 🎈
Balancing these types is super important for cells. Knowing these helps scientists and gardeners understand how to take care of plants and animals! 🌻🐾
Read Less
Mechanism of Osmosis
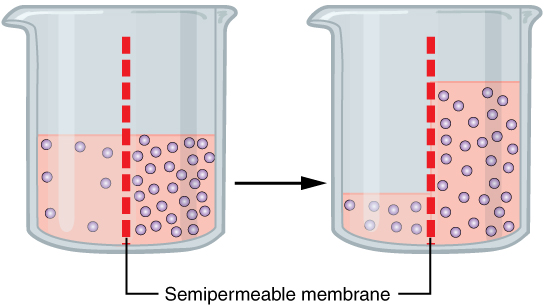
The magic of osmosis happens when water molecules decide to move! 🌊
Water molecules are tiny and love to travel. They’ll move through a selectively-permeable membrane, which acts like a filter. This membrane only lets certain things pass, allowing water but not larger stuff like salt. If you imagine two sides of a wall, where one side has many water molecules (a lot of water) and the other side has fewer (not much water), water will naturally go to the side with lesser water to help balance things out. It’s like a fun game of hide and seek with water! 🏃
♂️💦
Water molecules are tiny and love to travel. They’ll move through a selectively-permeable membrane, which acts like a filter. This membrane only lets certain things pass, allowing water but not larger stuff like salt. If you imagine two sides of a wall, where one side has many water molecules (a lot of water) and the other side has fewer (not much water), water will naturally go to the side with lesser water to help balance things out. It’s like a fun game of hide and seek with water! 🏃
♂️💦
Read Less
Definition of Osmosis
Osmosis is when water moves from one place to another through a special door called a selectively-permeable membrane. Imagine a sponge! 🧽
When you soak it in water, the water goes inside the sponge. In osmosis, water goes from an area with lots of it (like a puddle) to an area with less (like a dry sponge). This helps keep things balanced. Osmosis is important for all living beings, from tiny plants to big animals! 🌱🐘 By moving across membranes, water helps cells maintain the right amount of water and nutrients they need to live and grow!
When you soak it in water, the water goes inside the sponge. In osmosis, water goes from an area with lots of it (like a puddle) to an area with less (like a dry sponge). This helps keep things balanced. Osmosis is important for all living beings, from tiny plants to big animals! 🌱🐘 By moving across membranes, water helps cells maintain the right amount of water and nutrients they need to live and grow!
Read Less
Osmosis vs. Diffusion
Osmosis and diffusion sound alike, but they're a bit different! 🤔💡 Diffusion is the movement of particles (like tiny specks) from an area where they are crowded to where they are not. It’s like spreading out marbles on the floor. But osmosis specifically is about water moving through a selectively-permeable membrane! Imagine holding your breath and then taking a deep breath to fill your lungs; that’s diffusion! 🌬
️ In both cases, things want to move to balance out the concentration, but osmosis is all about the water’s journey! 🚶
♀️💦
️ In both cases, things want to move to balance out the concentration, but osmosis is all about the water’s journey! 🚶
♀️💦
Read Less
Osmosis in Plant Cells
Plants rely on osmosis to stay strong and healthy! 🌲
When plant roots soak up water from the soil, this water travels through their cells. If the roots are in a hypotonic solution (less salt), water enters the plant, making it stand tall and firm! 🌿
This is called turgor pressure. However, if there’s too much salt outside (hypertonic), plants can lose water and wilt! 😼
That’s why it’s important for gardeners to give plants the right amount of water with just enough nutrients to help them grow juicy and vibrant! 🍅🌻
When plant roots soak up water from the soil, this water travels through their cells. If the roots are in a hypotonic solution (less salt), water enters the plant, making it stand tall and firm! 🌿
This is called turgor pressure. However, if there’s too much salt outside (hypertonic), plants can lose water and wilt! 😼
That’s why it’s important for gardeners to give plants the right amount of water with just enough nutrients to help them grow juicy and vibrant! 🍅🌻
Read Less
Applications of Osmosis
Osmosis is not just a school topic but is used in many real-world scenarios! 🌎
For example, in food preservation, companies use osmosis to salt foods, drawing water out to help keep them fresh longer. In hospitals, doctors use osmosis to provide IV fluids to patients, making sure they get the right amounts of water! 🏥
Additionally, osmosis is vital in desalination plants, where saltwater is turned into clean drinking water. 💧
Scientists also study osmosis for agriculture, helping farmers grow better crops. Understanding osmosis helps improve health, food, and our environment! 🌾🌍
For example, in food preservation, companies use osmosis to salt foods, drawing water out to help keep them fresh longer. In hospitals, doctors use osmosis to provide IV fluids to patients, making sure they get the right amounts of water! 🏥
Additionally, osmosis is vital in desalination plants, where saltwater is turned into clean drinking water. 💧
Scientists also study osmosis for agriculture, helping farmers grow better crops. Understanding osmosis helps improve health, food, and our environment! 🌾🌍
Read Less
Osmosis in Animal Cells
Animals use osmosis too! 🐶
Our bodies are made up of tiny cells that need water to work properly. When we drink water, it enters our cells through osmosis. If a cell is in a hypotonic solution, it fills up with water, making it super happy! 🥳
But it’s different if the outside is hypertonic; the cell loses water and can shrivel like a raisin! 🍇
It’s crucial for us to stay hydrated, but too much salt can make us thirsty! So remember to drink water every day to keep our cells healthy! 🥤💧
Our bodies are made up of tiny cells that need water to work properly. When we drink water, it enters our cells through osmosis. If a cell is in a hypotonic solution, it fills up with water, making it super happy! 🥳
But it’s different if the outside is hypertonic; the cell loses water and can shrivel like a raisin! 🍇
It’s crucial for us to stay hydrated, but too much salt can make us thirsty! So remember to drink water every day to keep our cells healthy! 🥤💧
Read Less
Factors Affecting Osmosis
Many things can change how osmosis works! 🌞
First, the concentration of solutes (like salt or sugar) on each side of a membrane is really important. The bigger the difference, the faster osmosis happens! Second, temperature affects how fast water molecules move—warm water makes them race around! 🌡
️ Next, the type of membrane also matters; some membranes are better at letting water through than others. Finally, pressure can change osmosis; for example, squeezing water in a sponge can make it come out faster! All these factors help scientists understand how osmosis affects living things! 🤔🚀
First, the concentration of solutes (like salt or sugar) on each side of a membrane is really important. The bigger the difference, the faster osmosis happens! Second, temperature affects how fast water molecules move—warm water makes them race around! 🌡
️ Next, the type of membrane also matters; some membranes are better at letting water through than others. Finally, pressure can change osmosis; for example, squeezing water in a sponge can make it come out faster! All these factors help scientists understand how osmosis affects living things! 🤔🚀
Read Less
Common Misconceptions about Osmosis
Some people think osmosis and diffusion are the same, but remember—they’re different! 🚫
Osmosis only involves water moving through membranes, while diffusion is for all particles. Another misconception is that osmosis always makes things swell. While that's true in hypotonic solutions, in hypertonic solutions, things shrink instead! 😲
Lastly, some believe osmosis only happens in plants. Nope! Animals do it too! 🐢
Understanding these facts helps us learn better and appreciate the wonders of science! Keep asking questions and discovering the amazing world of osmosis! 🌏🔍
Osmosis only involves water moving through membranes, while diffusion is for all particles. Another misconception is that osmosis always makes things swell. While that's true in hypotonic solutions, in hypertonic solutions, things shrink instead! 😲
Lastly, some believe osmosis only happens in plants. Nope! Animals do it too! 🐢
Understanding these facts helps us learn better and appreciate the wonders of science! Keep asking questions and discovering the amazing world of osmosis! 🌏🔍
Read Less
Experimental Demonstrations of Osmosis
Want to see osmosis in action? 🧪
You can try a fun experiment! Take a potato and cut it into two pieces. Put one in fresh water (hypotonic) and another in saltwater (hypertonic). 🥔💦 After a few hours, the potato in fresh water will feel squishy and expand due to water entering, while the one in saltwater will shrink as it loses water! This is osmosis in action! 🌊
You can also use gummy bears! Place one in water and another in saltwater. Watch how they change size overnight! These experiments are entertaining and show how osmosis works! 🎉🌈
You can try a fun experiment! Take a potato and cut it into two pieces. Put one in fresh water (hypotonic) and another in saltwater (hypertonic). 🥔💦 After a few hours, the potato in fresh water will feel squishy and expand due to water entering, while the one in saltwater will shrink as it loses water! This is osmosis in action! 🌊
You can also use gummy bears! Place one in water and another in saltwater. Watch how they change size overnight! These experiments are entertaining and show how osmosis works! 🎉🌈
Read Less
Try your luck with the Osmosis Quiz.
Try this Osmosis quiz and see how many you score!
Q1
Question 1 of 10
Next
Explore More