Present
Facts for Kids
A chemical formula presents information about the proportions and types of atoms in a molecule, using element symbols and numbers.

Explore the internet with AstroSafe
Search safely, manage screen time, and remove ads and inappropriate content with the AstroSafe Browser.
Download
Inside this Article
Environmental Science
Dmitri Mendeleev
Carbon Dioxide
Information
Chemistry
Hydrogen
Bromine
Writing
Glucose
Formula
Oxygen
Did you know?
🧪 A chemical formula is like a recipe that shows how atoms combine to create different substances!
💧 The formula for water is H₂O, which means it has 2 hydrogen atoms and 1 oxygen atom.
📊 There are three main types of chemical formulas: empirical, molecular, and condensed!
🥗 Empirical formulas show the simplest ratio of atoms, like A₁G₂ for a fruit salad.
🍭 Molecular formulas give the exact number of each type of atom in a molecule, like C₆H₁₂O₆ for glucose.
🌈 Condensed formulas simplify chemical structures, showing how atoms are connected without drawing them out.
🌍 Chemical formulas are everywhere, like NaCl for table salt and CO₂ for carbon dioxide!
🔍 Even experienced scientists can make mistakes with chemical formulas, such as mixing up letters.
👩🔬 Chemical formulas help scientists predict how substances react with each other.
🌟 Understanding chemical formulas is essential for learning more about the world around us!
Show Less

Become a Creator with DIY.org
A safe online space featuring over 5,000 challenges to create, explore and learn in.
Learn more
Overview
Hey there, young scientists! 🌟
Have you ever wondered what makes everything around you? From water to candy, everything is made of tiny building blocks called atoms! A chemical formula is like a recipe that shows how these atoms combine to create different substances. For example, the formula for water is H₂O. This means every molecule of water has two hydrogen atoms (H) and one oxygen atom (O). Chemical formulas help scientists understand what things are made of and how they react with each other, just like how we follow cooking recipes to make yummy dishes! 🍽
️
Have you ever wondered what makes everything around you? From water to candy, everything is made of tiny building blocks called atoms! A chemical formula is like a recipe that shows how these atoms combine to create different substances. For example, the formula for water is H₂O. This means every molecule of water has two hydrogen atoms (H) and one oxygen atom (O). Chemical formulas help scientists understand what things are made of and how they react with each other, just like how we follow cooking recipes to make yummy dishes! 🍽
️
Read Less
Types of Chemical Formulas
There are three main types of chemical formulas: empirical, molecular, and condensed! 🧬
Each serves its own purpose. Empirical formulas show the simplest whole-number ratio of the atoms, like C₂H₄ becoming CH₂ for ethene. Molecular formulas show the actual number of each atom in a molecule, like C₆H₁₂O₆ for glucose. Lastly, condensed formulas write out atoms in a more compact format and show how they're connected! Each type helps scientists communicate information about compounds easily and accurately! 📊
Each serves its own purpose. Empirical formulas show the simplest whole-number ratio of the atoms, like C₂H₄ becoming CH₂ for ethene. Molecular formulas show the actual number of each atom in a molecule, like C₆H₁₂O₆ for glucose. Lastly, condensed formulas write out atoms in a more compact format and show how they're connected! Each type helps scientists communicate information about compounds easily and accurately! 📊
Read Less
What is a Chemical Formula?
A chemical formula uses symbols and numbers to represent a substance and describe what it’s made of! 🧪
For instance, in the formula H₂O for water, "H" stands for hydrogen, and "O" stands for oxygen. The little "2" tells us there are two hydrogen atoms. Chemical formulas help us know exactly which atoms are present and how many of each atom there are. This is super important for scientists when they study reactions or create new substances! Just like how knowing the correct ingredients is key to baking a cake! 🎂
For instance, in the formula H₂O for water, "H" stands for hydrogen, and "O" stands for oxygen. The little "2" tells us there are two hydrogen atoms. Chemical formulas help us know exactly which atoms are present and how many of each atom there are. This is super important for scientists when they study reactions or create new substances! Just like how knowing the correct ingredients is key to baking a cake! 🎂
Read Less
Condensed Formulas Explained
Condensed formulas are a neat way to write chemical structures without drawing the whole picture! 🌈
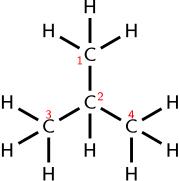
For example, instead of writing out the full structure of a molecule like butane, we can write it as CH₃(CH₂)₃CH₃. This notation tells us there are 4 carbon atoms and is a shorthand that still shows how the atoms are connected! Condensed formulas help scientists and students save time while grasping how a substance is structured. It's like a map of where every atom is and how they work together! 🗺
️
For example, instead of writing out the full structure of a molecule like butane, we can write it as CH₃(CH₂)₃CH₃. This notation tells us there are 4 carbon atoms and is a shorthand that still shows how the atoms are connected! Condensed formulas help scientists and students save time while grasping how a substance is structured. It's like a map of where every atom is and how they work together! 🗺
️
Read Less
Empirical Formulas Explained
Empirical formulas are like a shortcut to understand the basic ratio of different atoms in a compound! 📏
Imagine you made a fruit salad with 2 apples and 4 grapes. The empirical formula would just say you have 1 apple for every 2 grapes, which is written as A₁G₂. They don’t tell you the actual numbers of atoms but simplify the relationship. For example, benzene has the empirical formula CH, meaning there's 1 carbon atom for every hydrogen atom. Empirical formulas are super useful in chemistry! 🥗
Imagine you made a fruit salad with 2 apples and 4 grapes. The empirical formula would just say you have 1 apple for every 2 grapes, which is written as A₁G₂. They don’t tell you the actual numbers of atoms but simplify the relationship. For example, benzene has the empirical formula CH, meaning there's 1 carbon atom for every hydrogen atom. Empirical formulas are super useful in chemistry! 🥗
Read Less
Molecular Formulas Explained
The molecular formula gives the exact number of each type of atom in a molecule! 🍭
For example, the molecular formula for water is H₂O, which tells us there are 2 hydrogen atoms for each oxygen atom. For glucose, a sugary molecule, the formula is C₆H₁₂O₆, meaning it has 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms! This information is vital for scientists as it helps them understand how the substance behaves and how they might use it in reactions, baking, or even making candy! 🍬
For example, the molecular formula for water is H₂O, which tells us there are 2 hydrogen atoms for each oxygen atom. For glucose, a sugary molecule, the formula is C₆H₁₂O₆, meaning it has 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms! This information is vital for scientists as it helps them understand how the substance behaves and how they might use it in reactions, baking, or even making candy! 🍬
Read Less
Interpreting Chemical Formulas
Once you see a chemical formula, your scientist instincts kick in! 🌟
You can start figuring out what elements are in the substance by looking at the symbols. Remember the periodic table? It's the big chart of all known elements! The letters in a formula show which elements are used, and the numbers tell you how many there are. For example, in CO₂, “C” means carbon and “O” means oxygen, with the subscript “2” telling us there are two oxygen atoms. Interpreting formulas is a skill you’ll develop, making you a pro in chemistry! 🧙
♂️
You can start figuring out what elements are in the substance by looking at the symbols. Remember the periodic table? It's the big chart of all known elements! The letters in a formula show which elements are used, and the numbers tell you how many there are. For example, in CO₂, “C” means carbon and “O” means oxygen, with the subscript “2” telling us there are two oxygen atoms. Interpreting formulas is a skill you’ll develop, making you a pro in chemistry! 🧙
♂️
Read Less
Common Mistakes in Chemical Formulas
Even the best scientists can make mistakes with chemical formulas! 🚫
One common mistake is mixing up letters. “B” is for boron, while “Br” is for bromine! Sometimes, people forget to add numbers to show how many atoms there are, which can change the whole formula! For instance, writing H₂O instead of H₂O₃ gives very different results! Another mistake is skipping parentheses when needed. Always double-check your formulas! Just like in math or spelling, accuracy is super important when working with chemical formulas, ensuring you get the right answer every time! 🔍
One common mistake is mixing up letters. “B” is for boron, while “Br” is for bromine! Sometimes, people forget to add numbers to show how many atoms there are, which can change the whole formula! For instance, writing H₂O instead of H₂O₃ gives very different results! Another mistake is skipping parentheses when needed. Always double-check your formulas! Just like in math or spelling, accuracy is super important when working with chemical formulas, ensuring you get the right answer every time! 🔍
Read Less
Historical Development of Chemical Notation
Chemical notation has gone through some amazing changes! 🧐
In the early 1800s, a scientist named John Dalton introduced symbols for elements, helping everyone communicate better about chemistry. Later, Dmitri Mendeleev created the periodic table, organizing elements by their properties! The modern system of using letters and numbers to represent compounds was developed over time, with many scientists contributing like August Kekulé and his work on benzene. This teamwork helped create the shorthand we use today! Scientists have invented ways to express complex ideas simply, just like how we use emojis! 🧪
In the early 1800s, a scientist named John Dalton introduced symbols for elements, helping everyone communicate better about chemistry. Later, Dmitri Mendeleev created the periodic table, organizing elements by their properties! The modern system of using letters and numbers to represent compounds was developed over time, with many scientists contributing like August Kekulé and his work on benzene. This teamwork helped create the shorthand we use today! Scientists have invented ways to express complex ideas simply, just like how we use emojis! 🧪
Read Less
Examples of Chemical Formulas in Everyday Life
Chemical formulas are all around us in our daily lives! 🌍
For instance, the formula for table salt is NaCl, made of sodium (Na) and chlorine (Cl). The delicious carbon dioxide in fizzy drinks is represented as CO₂. You might be surprised to find out that white sugar has the formula C₁₂H₂₂O₁₁! Even the air we breathe contains nitrogen and oxygen, shown as N₂ and O₂, respectively! Knowing these formulas helps us understand the world’s basic elements and how they combine to create the things we love! 🍭
For instance, the formula for table salt is NaCl, made of sodium (Na) and chlorine (Cl). The delicious carbon dioxide in fizzy drinks is represented as CO₂. You might be surprised to find out that white sugar has the formula C₁₂H₂₂O₁₁! Even the air we breathe contains nitrogen and oxygen, shown as N₂ and O₂, respectively! Knowing these formulas helps us understand the world’s basic elements and how they combine to create the things we love! 🍭
Read Less
The Importance of Chemical Formulas in Science
Chemical formulas are super important in science because they tell us what substances are made of! 🧬
They help scientists design experiments, create new materials, and develop medicines. Knowing chemical formulas allows chemists to predict how substances will react with each other, just like understanding how ingredients interact when cooking! They also help in environmental science to study pollution and find ways to clean things up! Chemical formulas are the secrets to unlocking the mysteries of our world, making them essential for scientists and curious minds alike! 🌍🔍
They help scientists design experiments, create new materials, and develop medicines. Knowing chemical formulas allows chemists to predict how substances will react with each other, just like understanding how ingredients interact when cooking! They also help in environmental science to study pollution and find ways to clean things up! Chemical formulas are the secrets to unlocking the mysteries of our world, making them essential for scientists and curious minds alike! 🌍🔍
Read Less
Try your luck with the Chemical Formula Quiz.
Try this Chemical Formula quiz and see how many you score!
Q1
Question 1 of 10
Next
Explore More